The Kaz Lab
The Kaz Lab studies the interaction of light with molecules in the presence of magnetic fields.
Research in the Kaz Lab proceeds in two major directions: (1) spectroscopy and (2) chemical kinetics. The Kaz Lab is looking for new members to join an interdisciplinary research team. In addition to chemistry and biochemistry, there are opportunities for students in mathematics, physics, computer science and engineering to contribute. Interested students should contact Dr. Kazmierczak directly.
Magnetic Spectroscopy
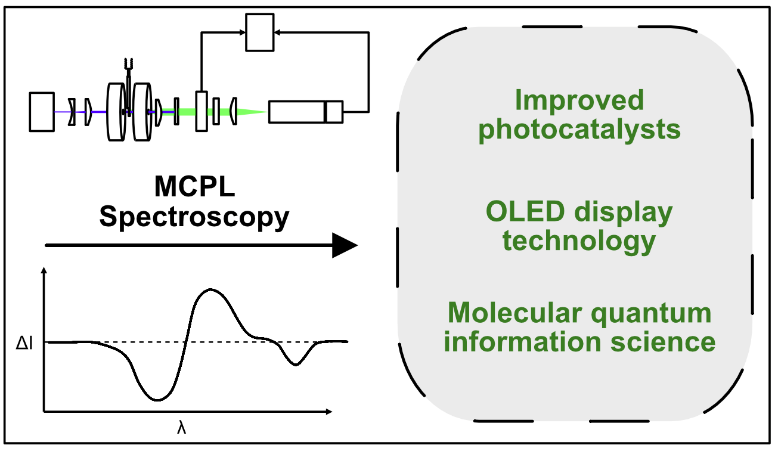 Spectroscopy is the part of chemistry that studies how light interacts with molecules.
Application of strong magnetic fields causes the usual light-matter interaction to
change in a detectable way. The change in the polarization of light yields valuable
clues about what the electrons are doing in the molecule. These insights can be leveraged
to (i) design better catalysts for pharmaceutical chemistry, (ii) improve the efficiency
of organic LED emitters used in display technology and (iii) design molecular systems
that process quantum information.
Spectroscopy is the part of chemistry that studies how light interacts with molecules.
Application of strong magnetic fields causes the usual light-matter interaction to
change in a detectable way. The change in the polarization of light yields valuable
clues about what the electrons are doing in the molecule. These insights can be leveraged
to (i) design better catalysts for pharmaceutical chemistry, (ii) improve the efficiency
of organic LED emitters used in display technology and (iii) design molecular systems
that process quantum information.
The Kaz Lab is building a unique type of instrument called a magnetic circularly polarized luminescence (MCPL) spectrometer. The instrument uses a laser beam to cause the molecules to fluoresce and cools the molecules to temperatures close to absolute zero using cryogenics. By analyzing the change in polarization of the emitted light, the nature of the excited state electronic wavefunction can be ascertained. Complementary experiments using magnetic circular dichroism (MCD) probe how the polarization of absorbed light is altered by magnetic fields. Very few variable-temperature, variable-field MCPL spectrometers exist, and the Kaz Lab is excited to apply this method to new scientific challenges.
The Kaz Lab has a range of spectroscopy projects suitable for all levels of students. Beginning students will gain experience in preparing samples for spectroscopy, working with transition metal catalysts, using a fluorimeter, and using the MCD and MCPL spectrometers. In addition to the above, advanced students will gain experience building and maintaining the magnetic spectroscopy instrumentation, setting up lasers and cryogenics, and using computational chemistry and group theory to interpret the spectra.
Chemical Reaction Network Kinetics
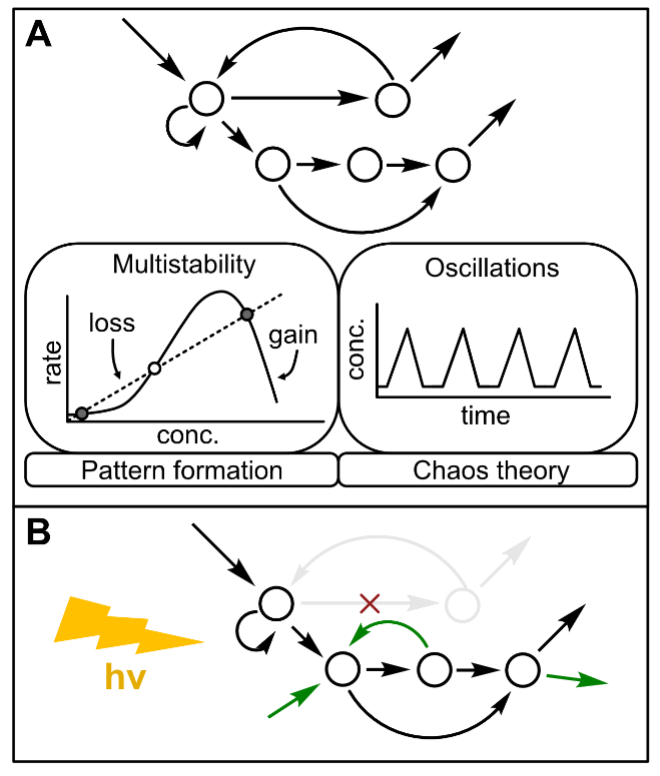 Chemical reaction networks (CRNs) have untapped potential for storing and processing
information. Before there was life capable of evolution, abiotic systems of chemical
reactions generated the information-containing building blocks of the first cell.
Furthermore, the “metabolism first” theory of the origins of life argues that self-propagating
CRNs, without genes or membranes, could have constituted the first proto-organisms
undergoing natural selection. If we can learn to find, analyze and design examples
of information processing in artificial small-molecule CRNs, we may be able to design
abiotic chemical systems displaying the responsive characteristics of living beings.
We can envision an age of smart chemical sensors exhibiting communal behaviors like
quorum sensing, soft materials capable of reproduction and self-healing, and devices
synchronized by an artificial circadian clock of chemical oscillations.
Chemical reaction networks (CRNs) have untapped potential for storing and processing
information. Before there was life capable of evolution, abiotic systems of chemical
reactions generated the information-containing building blocks of the first cell.
Furthermore, the “metabolism first” theory of the origins of life argues that self-propagating
CRNs, without genes or membranes, could have constituted the first proto-organisms
undergoing natural selection. If we can learn to find, analyze and design examples
of information processing in artificial small-molecule CRNs, we may be able to design
abiotic chemical systems displaying the responsive characteristics of living beings.
We can envision an age of smart chemical sensors exhibiting communal behaviors like
quorum sensing, soft materials capable of reproduction and self-healing, and devices
synchronized by an artificial circadian clock of chemical oscillations.
The functionality of CRNs comes not from the identity of their chemical products, but from their kinetics, i.e., how the concentrations of the various compounds evolve over time. The Kaz Lab is creating a new class of CRNs based on the use of light to activate/deactivate chemical reaction kinetics. While many chemical reactions are activated by applying heat, light can also be used as a reagent to overcome activation energy barriers. This area of research is known as photochemistry. Photochemical reactions can be carefully tuned by the amount, wavelength, duration and location of light applied. This enables precise control over the mechanistic details of the CRN kinetics. Just as the field of optogenetics revolutionized understanding of biological neural network function by using light to control individual neurons, so we expect photochemical control of small-molecule CRNs to produce new insights into the mechanistic requirements for life-like kinetic behavior.
Students will gain experience in analysis of chemical kinetics, spectrophotometry and flow reactors. In addition, students will learn to use mathematical tools from nonlinear dynamics to analyze the behavior of CRNs. Organic and inorganic synthesis may be involved in some projects.
A. Paul Schaap Science Center35 East 12th StreetRoom 3101Holland, MI 49423
workP. 616.395.7630
chemistry@hope.edu